Inside unconventional metal, electrons assume new shapes.
Redefining our understanding of the atom is one of quantum physics’ greatest accomplishments. Out came the small solar system created in the early 1900s, in which electrons circled a solid nucleus. Quantum physics, on the other hand, revealed that electrons had a much more intriguing existence, circling the nucleus in clouds that like small balloons. Atomic orbitals are the name for these balloon-like structures, which can be completely spherical, two-lobed, or clover-leaf shaped. How much the electron spins around the nucleus is shown by the number of lobes on the balloon.

That’s great for individual atoms, but when atoms combine to form a solid, like a piece of metal, for example, the outermost electrons in the atoms can link arms and lose sight of the nucleus from which they originated, resulting in a variety of oversized balloons that span the entire piece of metal. Instead of continuing to spin around their nuclei, they flow through the metal to transport electrical currents and release a variety of multi-lobed balloons in the process.
The first experimental proof that one metal—and likely others in its class—have electrons that manage to preserve a more interesting, multi-lobed structure as they move around in a solid has now been produced by researchers at the Quantum Materials Center (QMC) at the University of Maryland (UMD), working with theorists at the Condensed Matter Theory Center (CMTC) and Joint Quantum Institute (JQI). The researchers conducted an experimental analysis of these balloons’ shapes and discovered a complicated structure rather than a homogeneous surface. Not only is this strange metal intrinsically intriguing, but it could also be beneficial for creating noise-resistant quantum computers.
The researchers recently published their findings in the journal Physical Review Research.
“When I first discovered this, I was so excited,” says Hyunsoo Kim, a former postdoctoral researcher at QMC and lead author of the work. “But it took years to fully study, because it’s not a conventional concept and also experimentally very challenging to collect high-quality data.”
The scientists initially identified the metal in issue, yttrium platinum bismuth, or YPtBi, as a superconductor in 2011. At low enough temperatures, some substances turn into superconductors and completely lose their resistance to electrical current. Because it contains far less mobile, current-carrying electrons than other superconductors, YPtBi seemed an odd candidate for superconductivity. To the researchers’ astonishment, however, it nevertheless managed to become superconducting. Its behavior in the presence of a magnetic field further demonstrated that it wasn’t a typical superconductor.
The researchers came to the conclusion that the geometry of the electron orbitals was to blame at the time and that electrons with more angular momentum—that is, electrons that spin about themselves and trace out more circles in space—were creating an unheard-of condition of superconductivity.
“We had what I would call circumstantial evidence that the superconductivity is made up of these higher angular momentum electron pairs,” says Johnpierre Paglione, a professor of physics at UMD, the director of QMC, and the head of the experimental group in this collaboration. “But there was really no direct evidence of these high angular momentum electrons.”
The scientists increased the temperature and examined the substance in its regular, non-superconducting condition in the additional trials to provide more concrete proof. Then, they carried out a traditional measurement that depicts something resembling the aggregate atomic orbital for all the metal’s electrons.
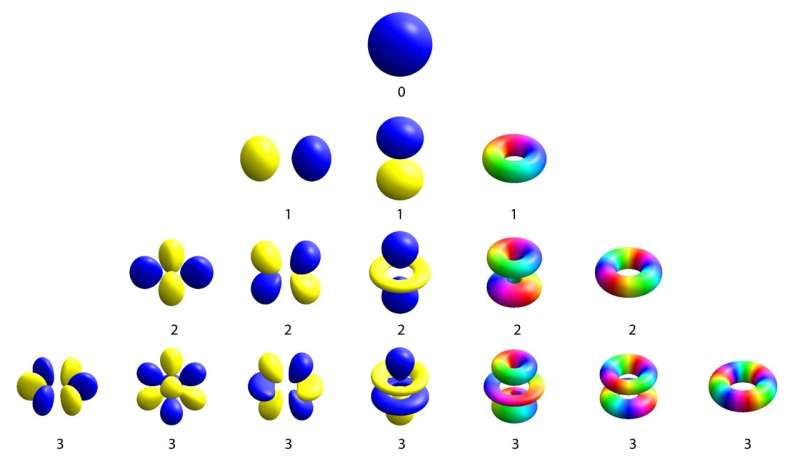
A crystal lattice, which is how atoms are organized when one looks inside a metal, is a tidy, repeating grid. The outermost electrons’ atomic orbitals merge together within crystals. As a result, the electrons can move freely throughout the metal and convey current far from their initial nucleus. It’s more typical to think about orbital balloons in this solid environment as a function of the speed and direction of the moving electrons rather than in space, where there are many enormous and cumbersome orbitals. The Fermi surface, a collective representation of atomic orbitals made up of the fastest-moving electrons in the crystal, is shaped like a balloon.
The Fermi surface’s form reflects the crystal’s underlying structure, which typically has little in common with the orbital structure of individual atoms. The Fermi surface, however, is not particularly large for materials like YPtBi that have a small number of mobile electrons. As a result, it still has some of the characteristics of the stationary electrons in the Fermi surface’s center.
According to JQI Co-Director and Fellow Jay Deep Sau, an associate professor of physics at the University of Maryland and a theoretical co-author on the new paper,
“it is rather cool and intricate that nature figures out counterintuitive atomic arrangements that allow the Fermi surface to retain signatures of the atomic orbitals.”
The scientists inserted a YPtBi crystal inside a magnetic field and monitored the current passing through the crystal as they adjusted the field in order to find this fascinating, paradoxical Fermi surface. They were able to map out the fastest electrons in each direction by varying the magnetic field’s direction. They discovered that the Fermi surface has a complicated form, with peaks and troughs along certain lines, similar to an atomic orbital with more angular momentum. The discovery of a more complex structure was unexpected given that the strong symmetry of the crystal would typically result in a more uniform, sphere-like Fermi surface.
This suggested that the collective electrons could be showing some atomic orbitals’ greater angular momentum characteristics.
The CMTC team really claimed to have made the first experimental observation of a high-angular momentum metal after theoretical calculations by the team revealed that the experimental data agreed with a high angular momentum model. The team issues a warning that even this experimental data might not be sufficient. The results of their measurements are influenced by the electrons’ effective mass and velocity dispersion in addition to the Fermi surface. The researchers showed in their study that it would be incredibly rare for these other variables to explain the observed peaks and troughs by methodically examining the angular dependency of these other quantities.
This greater angular momentum metal is fundamentally unique and may have uses in quantum computing. According to certain predictions, certain rare superconducting states might provide characteristics that are unaffected by the noise that occurs at any given time. These characteristics could be able to encode quantum bits, opening the door to the development of considerably more durable quantum computers. The current work is a key step in determining if YPtBi is exotic in the correct manner for this to happen, although it is not yet clear if it is.
“There’s many pieces to the puzzle of understanding exactly what kind of superconductor you have and whether you can exploit it to do quantum computation,” says Paglione. “There are some experimental challenges to get the rest of the pieces of the puzzle. But I think we’re a good chunk of the way there.”
Source:PhysOrg
Do not forget to share your opinion with us to provide you with the best posts !




0 Comments